Repair of Tendon Overuse Injuries
The Temenoff laboratory is interested in both knowing more about how ligaments and tendons are damaged, as well as designing new repair strategies for these tissues. In recent studies, we have used a rat model of an overuse injury to supraspinatus (rotator cuff) to demonstrate that damage occurs in the area of tendon insertion to bone. A novel multiplex zymography assay (developed in the Platt laboratory) was employed to determine activity of the cathepsin family of highly collagenolytic proteases. In overuse groups, increased cathepsin activity was seen in the insertion region, whereas little difference was observed in the remainder of the tendon. Moreover, collaborations with clinicians from Emory University allowed investigation of end stage disease (torn rotator cuff) in humans, where active cathepsins were also present. These results provide important information on a yet unexplored mechanism (protease-mediated ECM degradation) for tendon degeneration that may operate alone or in conjunction with other biochemical changes to contribute to chronic tendon degeneration leading to rotator cuff tear.
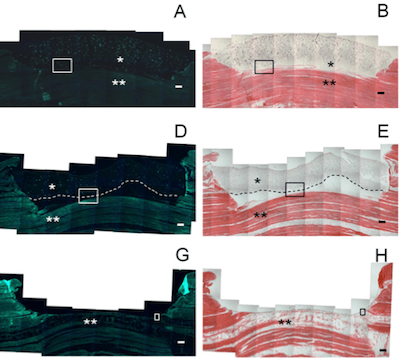
In terms of repair, MSCs have been suggested as a potential cell source for tendon/ligament tissue engineering to overcome the current limitation of lack of autologous fibroblasts. However, how best to deliver these cells to encourage engraftment remains unknown. In response, our laboratory has developed poly(ethylene glycol) hydrogel carriers with controlled degradation times due to altered susceptibility to hydrolysis. Three hydrogel formulations, including non-degrading, slower degrading (degraded in ~10 days) and faster degrading (degraded in ~5 days) hydrogels were selected for studies with MSCs in tendon tissue explants that had been treated with collagenase as a reproducible model of degenerate tendon. Quantitative analysis of the resulting histology images indicated that cell delivery from the hydrogels was dependent on the degradation rate. Based on these results, these hydrogels provide a versatile biomaterial platform to control cell delivery in injured tendons.